Research Highlights
UCSD Researchers Reveal Dynamic Behavior of Golgi Complex
November 2006
Researchers at UC San Diego reveal new details about the life cycle of the Golgi apparatus—the organelle responsible for correctly sorting, targeting, and trafficking protein in our cells. Constructing a novel protein tag suitable for light microscopy and correlated high-resolution electron microscopy, the team overcame intrinsic problems in observing the Golgi while undergoing cell division. The team of collaborators from UCSD’s National Center for Microscopy and Imaging Research and the Department of Pharmacology demonstrate a powerful new approach for observing the complex dynamics of the Golgi apparatus. Their work appeared online (13-Nov-2006) in advance of publication in the journal Proceedings of the National Academy of Sciences (PNAS).
Understanding the complex nature of the Golgi apparatus and what happens to it during cell division are important questions in cell biology. Along with the nucleus, mitochondria, and other key organelles, our cells would cease to function without the Golgi complex. And while cell biologists can readily follow the inheritance of the nucleus’ chromosomes, tracing the inheritance of the Golgi apparatus and the cell’s other organelles has been problematic.
The Golgi apparatus is often thought of as the distribution and shipping department for protein production in our cells because it edits raw proteins and assembles multi-protein complexes, originally synthesized elsewhere, for special delivery to other cell regions or export outside of the cell. Despite what is known, there are still many unanswered questions concerning how this organelle—with its complex architecture and critical role in trafficking proteins—is inherited from mother to daughter cells. Understanding how the Golgi functions is important as this organelle may be linked to Alzheimer’s disease and a host of other protein and lipid storage diseases that are likely brought about by the effects of incorrectly sorting proteins and lipids in our cells.
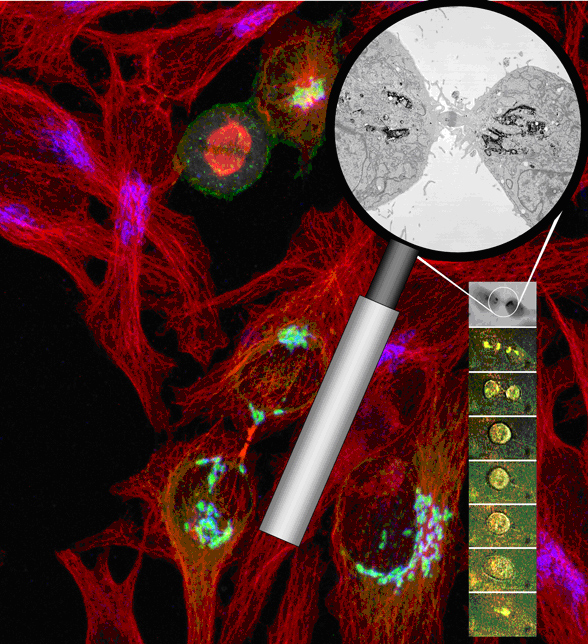
Familiar with the intrinsic difficulties that have limited previous studies of the Golgi, the research team designed a molecular probe optimized for correlated light and electron microscopic analysis and genetically fused it to a specific Golgi-resident protein, Mannosidase II. The fluorescent protein portion of the probe permitted the researchers to use light microscopy to trace the breakup and subsequent reassembly of the Golgi apparatus in living cells throughout mitosis.
To capture a live-cell preparation at a specific mitotic stage for high resolution electron microscopic analysis, the team fixed the specimen and used the excitation of the probe’s fluorescent protein to activate the polymerization and precipitation of a reducing agent, diaminobenzidine. Since the photoconversion or transfer of electrons from the fluorescing protein (donor) to the diaminobenzidine (acceptor) requires close spatial coupling, the electron-dense polymerization product is highly localized and decorates only the Golgi-resident marker. The resultant high specificity permitted the scientists to tag and delineate the Golgi’s fine structures using electron microscopy.
Using time-lapse live-cell imaging, the research team traced the dynamics of the Golgi apparatus through the cycle of cell growth (interphase) and cell division (mitosis). Following photoconversion of the same Golgi tag for correlated high-resolution electron microscopy, the team precisely located the Golgi proteins with high spatial and temporal resolution, revealing that the disassociated Golgi complex did not localize to the endoplasmic reticulum or other organelles—as previously thought—but maintained their autonomy throughout the cell’s life cycle.
These observations are refining our understanding of the Golgi’s dynamic behavior. For example, at telophase when a dividing cell’s chromosomes are being cordoned off into distinct new nuclei in the emerging daughter cells, the researchers observed—for the first time—the formation of four Golgi clusters or twins, two per daughter cell and documented the subsequent migration of the smaller twin to rejoin the major twin on the far side of the nucleus.
The successful approach used to study the Golgi apparatus has significance to future studies of other cell proteins and organelles. The powerful combinatorial probe described in this study can be readily re-targeted to other protein sequences and promises to provide cell biologists with a flexible tool for conducting correlated light and electron microscopic studies of other organelles.
Time-lapse movies tracing the fate of Golgi apparatus proteins throughout the life cycle of mammalian cells are available online through the PNAS Web site. (http://www.pnas.org/cgi/content/full/0608509103/DC1).
Citation: Guido Gaietta, Ben Giepmans, Thomas Deerinck, W. Bryan Smith, Lucy Ngan, Juan Llopis, Stephen R. Adams, Roger Y. Tsien, and Mark Ellisman (2006) Golgi twins in late mitosis revealed by genetically encoded tags for live cell imaging and correlated electron microscopy. Proceedings of the National Academy of Sciences (http://www.pnas.org/cgi/reprint/0608509103v1)