Research Highlights
Electron Microscopy Achieves A New APEX
Chemists from MIT together with NCMIR Scientists, Mark Ellisman, Tom Deerinck and Gina Sosinsky, have now designed a GFP equivalent for electron microscopy — a tag that allows scientists to label and visualize proteins with unprecedented clarity that offers much higher resolution than fluorescence microscopy. The new tag developed by Jeff Martell and Alice Ting, lead and senior authors of a paper describing the new tag in the Oct. 21 online edition of Nature Biotechnology, could help scientists pinpoint the locations of many cell proteins, providing new insight into those proteins’ functions. This new tag can label proteins throughout the cell — not only within mitochondria but also in the nucleus, the endoplasmic reticulum and the cytosol.
Dubbed APEX, the new tag is similar to naturally occurring proteins that have been tried as imaging labels for electron microscopy. Horseradish peroxidase (HRP) is one commonly used tag, but it works only in a few compartments of a cell. Other recently developed tags work throughout a cell but are technically challenging to use because they require light to be shined on the sample and oxygen to be bubbled through it. To improve on these methods, the researchers started with a protein similar to HRP, called ascorbate peroxidase (APX). APX is more versatile than HRP because it can function within a cell’s cytosol, in the main cavity of a cell. Both HRP and APX belong to a class of enzymes called peroxidases, which remove an electron and a proton from other molecules in a process known as oxidation. Every peroxidase has different targets, and one of HRP’s main targets is a molecule called DAB, which when oxidized can be visualized with electron microscopy. The researchers genetically engineered APX so that it would also target DAB. To use this new APEX tag (for “engineered APX”), the researchers deliver, into a living cell, a small ring of DNA containing the APEX gene joined to the gene for the protein they plan to image. The cell then produces the target protein, bound to the APEX protein. Next, the researchers need to deliver DAB, which is not normally found in cells. This delivery takes place during the process of “fixing,” or stabilizing cells, which must be done before they can be imaged with electron microscopy. When the APEX protein oxidizes DAB, it generates radicals that rapidly clump together into a tarlike polymer. That polymer can be detected through electron microscopy, allowing the researchers to pinpoint the location of the target protein.
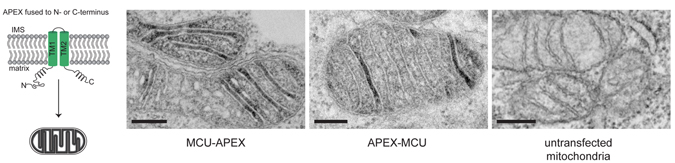
To demonstrate the usefulness of their new tag, the MIT and NCMIR researchers along with Yasemin Sancak and Vamsi Mootha of Harvard Medical School, and Thomas Poulos of the University of California at Irvine along with set out to resolve an open question regarding the location of a calcium channel protein discovered last year. Two research groups identified the protein and reported that it is located within mitochondria, but they had conflicting theories as to its precise location and orientation. Using the new imaging technique, the team labeled the protein and determined that it is embedded in the inner mitochondrial membrane and faces into the innermost part of mitochondria, the mitochondrial matrix.
Citation: Martell JD, Deerinck TJ, Sancak Y, Poulos TL, Mootha VK, Sosinsky GE, Ellisman MH, Ting AY (2012). Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nature Biotechnol 30:1143-1148. PMID: 23086203 (PMCID in process).
Adapted from http://web.mit.edu/newsoffice/2012/a-new-glow-for-electron-microscopy-1021.html
Online version of the article in Nature Biotechnology